-
CRGo World Conference and ICN Symposium 2023 concluded successfully on 29th November 20233rd December, 2023
The “Clinical Research Governance World Conference & International Clinical Trial Center Network Symposium 2023” co-hosted by HKU-CTC and the International Clinical Trial Center Network (ICN) concluded successfully on 29th November 2023. The conference was themed “Clinical Research Success through Good Governance” and brought together experts from Switzerland, Germany, United Kingdom, Austria, Australia, Nigeria, Japan, Singapore, Malaysia, Thailand, China, and Hong Kong to engage in in-depth discussions on various professional topics, including “International Collaboration in Clinical Research Ecology”, “Enhancement and Expansion of Research Capacity”, “Cutting-edge Research Methods” and “Regulatory Reform and Innovation”.
The conference attracted participants from over 40 countries and regions across six continents. The total number of attendees, including both onsite and online, exceeded 1,800. Distinguished speakers at the opening ceremony included Professor Lau Chak Sing, Dean of the LKS Faculty of Medicine at the University of Hong Kong, Professor Lo Chung Mau Lo, Secretary for Health of the Hong Kong SAR, Mr. Li Chuang, Deputy Director, Shenzhen Municipal Health Commission, and Dr med Christiane Blankenstein, Chairperson, International Clinical Trial Center Network (ICN).
Announcement on the Establishment of the “PRACTISE® Interactive Learning Centre for International Clinical Trials”In addition, HKU-CTC has annouced the establishment of the “PRACTISE® Interactive Learning Centre for International Clinical Trials” in Shenzhen. The goal of this learning centre is to nurture clinical research professionals for institutions and organizations involved in clinical research. Leveraging the unique PRACTISE® curriculum developed by HKU-CTC, participants will engage in diverse learning modules including lectures, case studies, group discussions, role-play, and hands-on training in a simulated environment to acquire core concepts, principles, and practical knowledge of international clinical trials.







From left: Dr. Desmond Yap, Medical Director, HKU Phase 1 Clinical Trials Centre; Mr Thomas Chan, Permanent Secretary for Health of HKSAR;
Mr Li Chuang, Deputy Director of Shenzhen Municipal Health Commission; Professor Lo Chung Mau, Secretary for Health of HKSAR;
Professor Lau Chak Sing, Dean of Li Ka Shing Faculty of Medicine of The University of Hong Kong; Dr Alvis Lo, Director of Health Bureau of Macao SAR;
Dr med Christiane Blankenstein, Chairperson of International Clinical Trial Center Network; Mr. Henry Yau, Managing Director of HKU-CTC -
25th Anniversary of HKU Clinical Trials Centre26th September, 2023
Attention all healthcare professionals and clinical trial enthusiasts! HKU Clinical Trials Centre is celebrating its 25th anniversary this year!
We are excited to announce that we will be hosting Clinical Research Governance (CRGo) World Conference 2023 & International Clinical Trial Center Network (ICN) Symposium 2023 on 29 November 2023 to celebrate this remarkable achievement. The theme of the Conference is “Clinical Research Success through Good Governance”. Speakers around the world including UK, Switzerland, Germany, Australia, Malaysia, Singapore, Mainland China and Hong Kong SAR will share the latest trends, challenges and opportunities in the field of clinical trials. You may refer to the programme here: https://25anniversary.ctc.hku.hk/crgo2023/
Besides, we are proud to host the HKU-CTC Silver Jubilee Ceremony & Gala Dinner at Holiday Inn Golden Mile Hong Kong on the same day.
Stay tuned for more registration details. We can't wait to celebrate with you and look forward to seeing you there!

-
New Project Form for Sponsored Clinical Studies25th September, 2023
The University of Hong Kong Clinical Trials Centre (HKU-CTC) has launched a new webpage for electronic new project form (e-NPF) submission for sponsored clinical studies (https://enpf.hkuctc.com/). The webpage serves as the central platform for managing and coordinating clinical studies under HKU and Queen Mary Hospital, and is a significant step towards streamlining the process of clinical study preparation.
This new page is a breakthrough for clinical investigators, research organizations, and the healthcare industry. It allows organizations to request research administration services, such as initial ethics submission, budget calculation, and contract preparation from HKU-CTC, all in an electronic form. The information provided on the form will be used for submissions to the relevant Institutional Review Boards (IRBs). By submitting the form to HKU-CTC, organizations warrant that the information provided is correct and accurate to the best of their knowledge. We hope that the launch of this new webpage will help us better serve our partners at large.
-
Explore the world of clinical research at the HKUMedify Summer Immersion Programme31st July, 2023
On July 31, 2023, some 120 secondary 5 students had a half-day journey to explore the world of clinical research with HKU Clinical Trials Centre. They engaged in a wide range of job tasting activities and had the opportunity to tour the HKU Phase 1 Clinical Trials Centre.
Their exploration began with an introduction to clinical trials and the drug discovery process, highlighting the importance of clinical trials in bridging medications from laboratory to clinical use. Next, the students gained some hands-on experience to evaluate the ethical dilemmas of clinical studies which might be encountered in real-life. Following that, they utilized their generation Z talents to create mock Instagram posts to recruit study volunteers – and their work was impressive!


-
Secondary School Students Learn about Clinical Trials During Visit to HKU Phase 1 Clinical Trials Centre29th June, 2023
On February 23, 2023, a group of secondary school students were given a rare opportunity to visit the HKU Phase 1 Clinical Trials Centre. They learned about the importance of ethical considerations, volunteers’ safety and data integrity, as well as the role that clinical trials play in advancing medical knowledge and improving patient care. As the students toured around the facility, they were impressed by the level of details put into the Centre’s design for safety and accuracy. The visit highlighted the importance of clinical trials in advancing medical knowledge and left a lasting impression on the students, inspiring them to consider careers in medical research and healthcare.

-
Conference on “Governance of Medical AI” on May 9-10, 20238th May, 2023
HKU Centre for Medical Ethics and Law is organizing an international conference on “Governance of Medical AI ”, which is supported by HKU-CTC.
Date: May 9-10, 2023 (Tue-Wed)
Venue: Cheng Yu Tung Tower, Centennial Campus, HKU or via Zoom
Registration: Please click here
The conference will address the regulatory and ethical challenges posed by medical devices with AI capabilities and their applications in genomic medicine. It will focus on governance policies of the National Medical Products Administration of China, the European Medicines Agency and the US Food & Drug Administration. Prominent speakers and experts from around the world will share their insights and expertise on various topics.
 Click here to zoom.
Click here to zoom. -
Clinical Research Trainee Programme 2023 – First-round Application Opens Now!15th March, 2023
Following the success last year, we are pleased to announce that the Clinical Research Trainee Programme 2023 is open for application now!
This 12-month programme provides graduates with a distinctive opportunity to acquire valuable experience and training in diverse aspects of the clinical research industry. Our well-structured and comprehensive learning scheme encompasses international clinical research standards, research technical skills, and soft skills to ensure a well-rounded experience. For more details, please refer to: https://www.hkuctc.com/career.php
If you are interested in the programme, you are invited to join the Recruitment Talk. We will share with you the programme details, and the trainees of last year will share with you the experience working at HKU-CTC. Details are as follows:-
Date: March 29, 2023 (Wednesday)
Time: 5:30pm – 6:30pm
Mode: Zoom
Medium: Cantonese
Register now and secure your seat!
-
Alibaba Cloud Summit Hong Kong 202219th December, 2022
Mr. Henry Yau, Managing Director of HKU-CTC, was invited as a keynote speaker to give an introduction on the topic of “Health. Research. Digital Innovation.” at the Alibaba Cloud Summit Hong Kong 2022 on November 29, 2022. The summit - with the theme of “Sustainable Innovation” - aimed to facilitate the participants’ understanding of digital innovation in different industries. Henry shared his insight on how digital innovation helps the biomedical industry and clinical institutions accelerate novel drug development, with special highlight on the application of cloud technology on creating a digital platform for clinical trials institution management and enhancing cross-border collaboration in the Greater Bay Area.


-
International Clinical Trial Center Network – Newsletter Issue 1525th August, 2022
We are pleased to share with you the Issue 15 of Newsletter issued by International Clinical Trial Center Network (ICN):-
https://www.icn-connect.org/newsletter/1232The featured articles of this issue is about “Decentralised Clinical Trials”. The outbreak of COVID-19 has disrupted many clinical studies. A separate side-effect of the pandemic has been swift adoption of virtual interactions between physicians and patients to provide continuity of care while maintaining social distancing. Therefore, interest in decentralized clinical trials (DCTs) that use “virtual elements” has grown in parallel with acceptance of “virtual medicine”, accelerating shifts in clinical trial design that many feel are long overdue.

-
HKU medical research team starts large-scale clinical trial on COVID-19 Vaccine (Omicron Variant)2nd June, 2022
A medical research team from the University of Hong Kong (HKU) started a large-scale clinical trial on a new COVID-19 inactivated vaccine (Omicron variant). The study is led by Professor Ivan Hung, Chief of Division of Infectious Diseases and Clinical Professor of Department of Medicine, LKS Faculty of Medicine, the University of Hong Kong (HKUMed) and HKU Clinical Trials Centre (HKU-CTC) is responsible for coordination and technical support.
A press conference was held on 31st May, 2022 to share with the public more about the details of the clinical trial. Professor Ivan Hung, Dr. Kelvin To (Chairperson and Clinical Associate Professor of Department of Microbiology, HKUMed) and Mr. Henry Yau (Managing Director of HKU-CTC) were invited. For more details about the press conference, please visit: https://hku.hk/press/news_detail_24616.html
As thousands of volunteers are needed for this study, if you are interested to join, please register via the link below:
https://redcap.link/joinctc2298


-
HKU/QMH completed registration on the newly launched Clinical Trials Institution Registration Management Platform (藥物臨床試驗機構備案管理信息平台) of NMPA on 13 clinical specialties/areas4th April, 2022
HKU/QMH has successfully completed the registration on the Clinical Trials Institution Registration Management Platform (藥物臨床試驗機構備案管理信息平台) in Mar 2022 on 13 clinical specialties, including:-
- Cardiology
- Endocrinology & Metabolism
- Gastroenterology & Hepatology
- Haematology & Bone Marrow Transplantation
- Hepatobiliary & Pancreatic Surgery and Liver Transplantation
- Nephrology
- Neurology
- Obstetrics & Gynaecology
- Oncology
- Orthopaedics & Traumatology
- Paediatrics & Adolescent Medicine
- Respiratory Medicine
- Phase 1 Clinical Trials Centre
HKU/QMH has been accredited by the NMPA for conducting clinical drug trials in six clinical specialties since 2006. HKU Phase 1 Clinical Trials Centre and more clinical specialties were further accredited in July 2016. According to the latest requirements and guidelines of the National Medical Products Administration (NMPA) of China, clinical trial sites and the principal investigators which conduct a study filed with the NMPA should registered on the Clinical Trials Institution Registration Management Platform (藥物臨床試驗機構備案管理信息平台). The listed investigators can act as the principal investigator for the NMPA-filed study, and the clinical drug trial data from these specialties are acceptable by the NMPA for evaluation of new drug approval applications.
-
International Clinical Trial Center Network – Newsletter Issue 142nd February, 2022
We are pleased to share with you the Issue 14 of Newsletter issued by International Clinical Trial Center Network (ICN):-
https://www.icn-connect.org/newsletter/1145
The featured articles of this issue is about “The New Normal after COVID-19”. COVID-19 has changed the way we live our lives. At first, we were wishing the pandemic could be over soon and the world could assume normal. Two years passed by and we are still wearing masks and the scientists are striving hard to figure out how to combat the virus. The world has shifted, but it is not completely on the bad side. You can read more about how the ICN members worldwide learn to adapt to the new normal.

-
PRACTISE®IIS - Principles and Practice of Clinical Research Data Management and Electronic Data Capture10th January, 2022
To prepare our colleagues for enjoying a free-of-charge IISupreme EDC service supported by HKUMed, another course of the PRACTISE® IIS training - Principles and Practice of Clinical Research Data Management and Electronic Data Capture - was successfully held on 15th & 16th December, 2021 in form of a webinar. Mr. Chi-Tao Ng, our Assistant Director, Project Strategy & Management and Mr. Mark Hui, our Medical Statistician, shared their experience in data management for IISs. The course attracted about 100 attendees. The course materials and the free IISupreme EDC service will be soon available on IISupreme.


-
HKU-CTC Long Service Award Presentation Ceremony30th December, 2021
HKU-CTC Long Service Award Presentation Ceremony was held on December 22, 2021. 23 staff members received awards that honoured their dedicated service. History of CTC was also shared at the ceremony to celebrate the development and success of the centre throughout the past years. Mr. Henry Yau, Managing Director of HKU-CTC who has served the centre for over 25 years himself, thanked the staff for their contributions, shared his reflection on working at CTC, and encouraged colleagues to keep up with the great synergy in the future.



-
International Clinical Trial Center Network (ICN) Steering Board Meeting and Annual General Meeting 202130th November, 2021
The ICN Annual Steering Board Meeting 2021 took place on November 16, 2021 in the form of virtual meeting and was hosted by Cambridge Clinical Trials Unit (CCTU), Cambridge University Hospitals NHS Foundation Trust, one of the steering board members.
During the meeting, all steering board members made decisions on key governance items. Mr. Henry Yau, ICN Chairperson cum Managing Director of HKU-CTC and Ms. Dr med Christiane Blankenstein, ICN Vice-Chairperson cum Director of Muenchner Studienzentrum, led the discussion on the strategic plan of ICN for the incoming year, which set out a direction for the Operations Team to work on in the year to come.
The ICN Annual General Meeting 2021 was held on November 17, 2021. Three experience sharing sessions were arranged and representatives from Cambridge University Hospitals and AstraZeneca were invited to talk about patient and public involvement as well as the influences of COVID-19 on clinical trials, which are both hot topics in 2021. Enthusiastic responses were received during the Q&A session.
The next ICN Steering Board Meeting and Symposium will be hosted by Muenchner Studienzentrum, Technical University Munich, School of Medicine, in October 2022.
-
“A tour to HKU Phase 1 Clinical Trials Centre” Interview by Our Hong Kong Foundation5th November, 2021
Curious about our HKU Phase 1 Clinical Trials Centre? In the episode 1 of the interview, you will have a view of the Centre, and Mr. Henry Yau, our Managing Director, will also introduce HKU’s efforts and achievements in clinical research and novel drugs development.
https://www.youtube.com/watch?v=IWzwTAenZzA
In episode 2, you will know more about the strengths of Hong Kong in conducting clinical trials, as well as the challenges we need to overcome.
https://www.youtube.com/watch?v=LsT8brGP_F4

-
International Clinical Trial Center Network – Newsletter Issue 1325th August, 2021
We are pleased to share with you the Issue 13 of Newsletter issued by International Clinical Trial Center Network (ICN).
https://www.icn-connect.org/newsletter/1055
The topic of the featured articles of this issue is about “Challenges under COVID-19”. The outbreak of COVID-19 has brought about unprecedented impact to every aspect of people’s lives. In this newsletter, ICN members share the impacts by COVID-19 and how they tackled the challenges.

-
New Regulations about Advanced Therapy Products under Pharmacy and Poisons (Amendment) Ordinance 2020, effective from August 1, 202130th Jul, 2021
The Amendment Ordinance introduces a clear regulatory framework for Advanced Therapy Products (ATPs) which cover gene therapy products, somatic cell therapy products and tissue engineered products. After the Amendment Ordinance comes into operation, ATPs will form a specific subset of pharmaceutical products under the Pharmacy and Poisons Ordinance (Cap. 138). As such, requirements for pharmaceutical products under Cap.138 and other relevant ordinances will apply to ATPs. These include registration prior to marketing, obtaining prior approval for conducting clinical trials, licensing of manufacturers and distributors, and import/export control. Besides, there will be additional requirements on labelling and record keeping specific to ATPs to enhance traceability of the products.
To help stakeholders better understand the enhanced regulatory framework for ATPs, please read the guidance documents relevant to ATPs prepared by the Pharmacy and Poisons Board of Hong Kong (https://www.ppbhk.org.hk/eng/regulation_of_atp.html) and the Drug Office of Hong Kong Department of Health (https://www.drugoffice.gov.hk/eps/do/en/pharmaceutical_trade/atp_regulation.html).
-
IISupreme - Investigator-Initiated Clinical Study One-Stop Platform Stage 1 Launched!15th Jun, 2021
Investigator-initiated clinical studies (IISs) play an important role in translating medical discoveries and hypotheses into clinical practice and bringing important value to advancement of human healthcare. To encourage and facilitate more high impact IISs, HKUMed has delegated the Clinical Trials Centre (HKU-CTC) to establish an ‘IIS Facilitation Scheme’, through which a range of supports and services will be offered to clinical investigators.
IISupreme (https://iis-ctc.hku.hk/) is an Investigator-Initiated Clinical Study One-Stop Platform dedicated to supporting investigators and clinical research personnel by stages.
- Stage 1 is now launched to provide PRACTISE®IIS training and useful resources for clinical research.
- Stage 2 will be launched by mid of year for users to request services via the platform including clinical trial insurance management and research agreement administration. An electronic data capture platform, the REDCap, will be made available as a special feature!
PRACTISE®IIS is a comprehensive clinical research training programme comprises 4 courses.
- The first two courses - ‘Study Design & Sample Size Calculations’ and ‘Ethics, Regulatory & Legal Compliance’ - were completed as webinars with great success and are now available via IISupreme.
- HKU-CTC will continue to organize courses on IISs. Announcements will be made via IISupreme, HKU-CTC’s website (https://www.hkuctc.com/) and HKU emails.

-
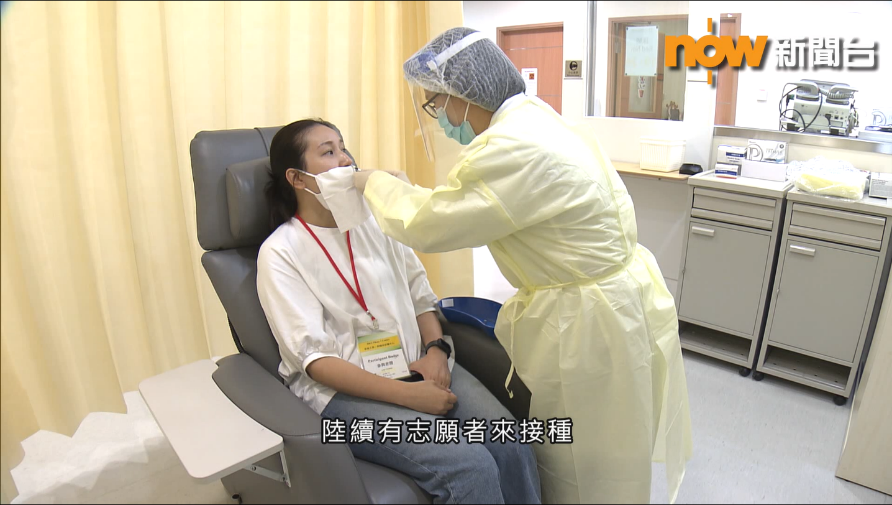
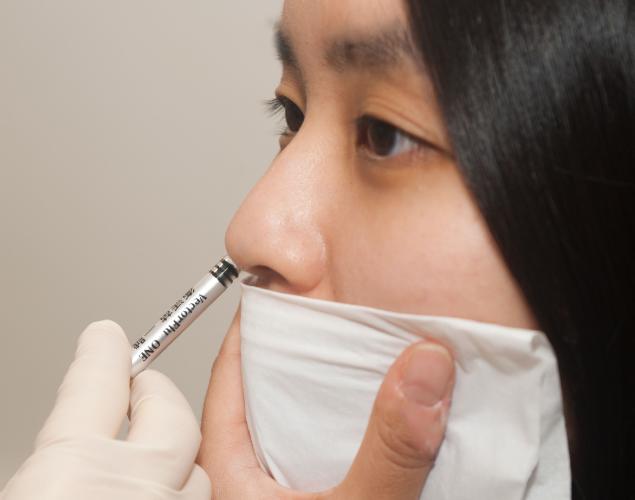
“Phase 1 Clinical Trial of VectorFlu® ONE” Interview by Now News6th May, 2021
Now News visited HKU Phase 1 Clinical Trials Centre earlier to produce a report on the phase 1 clinical trial of VectorFlu® ONE. Check it out here:-
-
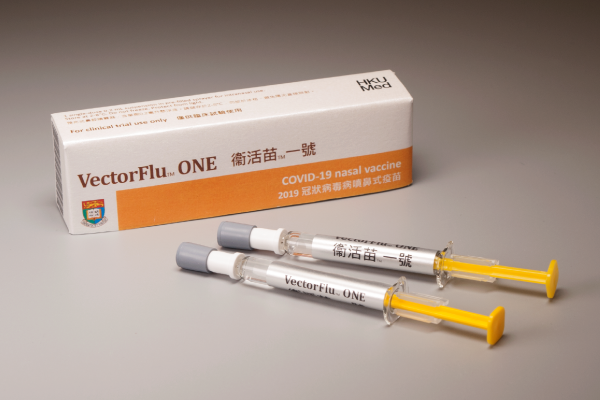
Phase 1 clinical trial of VectorFlu™ ONE, COVID-19 nasal vaccine, has commenced!14th May, 2021
The phase 1 clinical trial of VectorFlu™ ONE, the world’s first COVID-19 nasal vaccine developed by the research team of HKU Department of Microbiology, has commenced in March 2021 at the HKU Phase 1 Clinical Trials Centre.
VectorFlu™ ONE is the first novel vaccine that is developed by Hong Kong and goes into human trial. The vaccine technology is recognized by the China government as one of the few COVID-19 vaccine technology platforms. This phase 1 clinical trial is supported by the Health and Medical Research Fund of Hong Kong (HMRF) and Coalition for Epidemic Preparedness Innovations (CEPI), and is managed in accordance with the highest international ethics, regulatory and industrial standards.
-
PRACTISE® IIS: Training Course on Ethics, Regulatory and Legal Compliance14th Jan, 2021
The second course of the PRACTISE IIS training series was successfully held on January 14, 2021 in form of an online symposium. The theme of this course was “Ethics, Regulatory & Legal Compliance” relevant to investigator-initiated clinical studies. In addition to speakers from HKU-CTC, Mr. Daniel Li and Mr. Danny Yeung, Senior Director and Director of Gilead Hong Kong, were also invited to share their practical experience in the regulatory process and requirements for coordinating clinical studies in mainland China. The course attracted enthusiastic response from over 100 attendees.

-
Successful completion of a bioequivalent study of fenofibrate (a generic drug)29th Dec, 2020
HKU-CTC successfully completed a clinical study of fenofibrate (a generic drug) before the fourth wave of COVID-19 outbreak.
The bioequivalent study which required 52 healthy volunteers was conducted in the HKU Phase 1 Clinical Trials Centre in Queen Mary Hospital. Aiming to finish the clinical study before a possible fourth wave of COVID-19 outbreak in Hong Kong, CTC decided to kick start and expedite the study in mid-September with an ambitious timeline. Thanks to the team’s effective coordination and the volunteers’ support, it took only two and a half months from pre-screening of volunteers to the final study visit on 26 November, 2020 that marked the completion of the study.
CTC is devoted to facilitating clinical studies while taking volunteers’ safety and well-being as the priority, and will continue to foster healthcare innovation with professionalism and efficiency amid the pandemic.

-
The Hong Kong Association of the Pharmaceutical Industry GBA Training 202015th Dec, 2020
Mr. Henry Yau, Managing Director of HKU-CTC, was invited to speak on the topic of “Development of Clinical Trials in the Greater Bay Area: Overview, Challenges and Opportunities” at the GBA Training of The Hong Kong Association of the Pharmaceutical Industry on 2 December, 2020. The online training aimed to facilitate the participants’ understanding of the healthcare and pharmaceutical industry in the Greater Bay Area. Mr. Yau gave an overview of the current clinical trial environment in GBA, and shared his insights on the important roles of the Hong Kong pharmaceutical industry and clinical research professionals in the strategic and synergistic development.

-
Training Symposium on Research Ethics & Integrity for Students of the MSc Programme in Nutrition and Healthy Ageing at The Hong Kong Polytechnic University4th Dec, 2020
The value of clinical studies relies on their credibility, which is founded on research ethics and integrity. Community clinical studies for elderly service, in particular, require special ethical considerations to safeguard the rights, safety, and well-being of the vulnerable, aged volunteers.
To equip students with knowledge of updated views on the principles and practice of clinical research ethics and good clinical practice, a training symposium was held by HKU-CTC for students of the MSc Programme in Nutrition and Healthy Ageing at The Hong Kong Polytechnic University on 28 November, 2020 upon invitation of the university.
Mr. Henry Yau and Dr. Creany Wong, HKU-CTC’s Managing Director and Deputy Managing Director respectively, shared their insights on clinical research and clinical research ethics, principles and practice of ICH GCP, and special legal and ethical considerations in community clinical research. They also led discussions on practical case studies on nutritional products in Hong Kong to help participants translate theories into practice.

-
The International Clinical Trial Center Network (ICN) Steering Board Meeting and Annual General Meeting 202026th Nov, 2020
HKU-CTC successfully hosted the ICN Steering Board Meeting and Annual General Meeting on 24-25 November, 2020. Participants engaged actively and had vivid discussions – even though the meetings were moved online due to the pandemic.
At the Steering Board Meeting, Mr. Henry Yau, Managing Director of HKU-CTC, was elected the new ICN Chairperson. He will work with the new Vice Chairperson, Dr. Christiane Blankenstein of Munich Study Center, Technical University of Munich, the ICN Steering Board, and the ICN Operations Team to contribute to the further development of ICN in the coming two years. The Steering Board also formulated a strategic plan towards realization of the values of “Global, Excellence, Harmonization, Impacts”. The Annual General Meeting was held on 25 November, 2020 with 50 representatives from 19 institutions around the world. Representatives discussed within work groups and reported their collective insights at the meeting.
The next ICN Steering Board Meeting and Annual General Meeting will be hosted by Cambridge Clinical Trials Unit (CCTU), Cambridge University Hospitals NHS Foundation Trust in November 2021.
-
New Public Information Platform of Clinical Trials Centre3rd Nov, 2020
In order to raise public awareness on clinical development of new medications, devices and medical technologies, we are delighted to announce the launch of the “HKU Clinical Trials Centre Public Information Platform” (港大臨床試驗中心公眾資訊平台). This open, online platform offers you clinical scientific information and news in an interesting and easily understandable manner, and we wish it will help answer questions you may have in mind about clinical research and clinical trials.

-
Training Course on Study Design & Sample Size Calculation for Investigator-initiated Clinical Studies on 28th August, 202015th Sep, 2020
Investigator-initiated clinical studies (IISs) play an important role in translating medical discoveries and hypotheses into clinical practice and bringing important value to advancement of human healthcare. To encourage and facilitate more high impact IISs, HKUMed has recently delegated the Clinical Trials Centre (CTC) and the Biostatistics and Clinical Research Methodology Unit (BCMU) to establish an “IIS Facilitation Scheme”, through which a range of supports and services will be offered to clinical investigators by stages. The first initiative under this scheme is a comprehensive training programme (namely “PRACTISE® IIS”) which comprises 4 courses. The first course – Study Design & Sample Size Calculations – has completed by way of an online seminar on 28th August, 2020 with great success. Professor Ian Wong, Head of Department of Pharmacology and Pharmacy, and Dr. Helen Zhi, Director of BCMU, shared their practical tips and experience in designing and managing big data research and clinical trials with over 300 participants and received enthusiastic response. CTC will continue to organize courses on IISs. Announcements will be made via HKU emails and CTC’s website (https://www.hkuctc.com).

-
NMPA issues new clinical trials regulations and guidelines30th Jun, 2020
The National Medical Products Administration (NMPA) of China has recently issued the new version of “Good Clinical Practice (藥物臨床試驗質量管理規範)” and “Guidelines on Retention of Essential Documents for the Conduct of a Clinical Trial (藥物臨床試驗必備文件保存指導原則)”, which will come into effect on 1st July, 2020. These new documents were developed following China’s participation as a regulatory member of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) in 2017, and therefore are highly harmonized with the ICH standards. Details are available on the NMPA website:
-
Clinical Studies of COVID-195th March, 2020
To help identify possible treatments for tackling COVID-19, The University of Hong Kong Clinical Trials Centre join forces with Queen Mary Hospital, Prince of Wales Hospital, Princess Margaret Hospital and the manufacturer of remdesivir, an investigational antiviral drug, in arranging for two clinical trials targeting for the treatment of moderate to severe COVID-19 patients. With a common goal of accelerating the preparation for the trials, the parties have been working hard in the past few weeks and quickly obtained the approvals from the research ethics committees. It is anticipated that the trials will be initiated within this month (i.e. March 2020).

-
Celebrating Chinese New Year with Clinical Trial Supporters15th February, 2020
HKU-CTC set up a Chinese New Year Booth at Queen Mary Hospital on 21st January, 2020, to celebrate Chinese New Year with the supporters. Dr. Hong, one of the CTC Panda Ambassadors, visited the booth on that day. He gave out calendar cards and fai-chuns to the visitors. Visitors who participated in the quiz game at the booth and answered all questions correctly were given specially designed red packets as well. The responses were enthusiastic. Here are some highlights of the event:




-
Annual Academic Meeting of Drug Clinical Evaluation Professional Committee of the Chinese Pharmaceutical Association21st November, 2019
Mr. Henry Yau, Managing Director of HKU-CTC, and Dr. Creany Wong, Associate Director of HKU-CTC, were invited to speak at the Annual Academic Meeting of Drug Clinical Evaluation Professional Committee of the Chinese Pharmaceutical Association during October 19-20, 2019 in Beijing. The meeting was attended by hundreds of the professionals in the industry. They presented the topic of “International Collaboration on Academic Clinical Research”. During the session, International Clinical Trial Center Network (ICN)’s mission, activities and future developments were introduced. The strong interest of the participants attracted another invitation to present in another meeting in Changzhou, which will involve representatives from the top-notch clinical trials centres designated by the Chinese government (aka. Centres of Excellence).


-
International Clinical Trial Center Network (ICN) Annual Steering Board Meeting and Symposium 201918th October, 2019
The ICN Annual Steering Board Meeting 2019 took place on September 26, 2019 in Freiburg, Germany and was hosted by Clinical Trials Unit Freiburg of University Hospital Freiburg, one of the founding members. During the meeting, all steering board members reported their recent activities, and together formulated an action plan for the coming year through intensive small group discussions and open exchange of ideas over panel meetings. Mr. Henry Yau, Managing Director of HKU-CTC, was elected the new ICN Vice-Chairperson and will work together with the new Chairperson, Dr. Thomas Hiemstra of Cambridge Clinical Trials Unit and other Steering Board members to contribute to ICN’s exciting development in the coming two years.
The ICN Symposium 2019 was held following the Steering Board Meeting. Twelve speakers from Germany, United Kingdom, Switzerland, Sweden, Turkey, Taiwan and Hong Kong shared their insightful ideas around the theme of “Clinical Trials in the Global and Digital World”. Mr. Henry Yau, our Managing Director, and Dr. Creany Wong, our Associate Director, shared their experience on “clinical trials central management and digitalization” and received enthusiastic responses from the floor.
The next ICN Steering Board Meeting and Symposium will be hosted by HKU-CTC in November 2020 in Hong Kong.




-
HKU Phase 1 Clinical Trials Centre opens door for newly admitted MBBS students2nd August, 2019
When most people think of medical careers, the life of a practicing doctor immediately come to mind. However, there is also a very different option in the medical field which involves an alternative pathway: be a medical researcher.
30 students who are newly admitted to the HKU Bachelor of Medicine and Bachelor of Surgery Programme (MBBS) this year visited HKU Phase 1 Clinical Trials Centre on July 8, 2019. An information session introducing the concepts of clinical research was delivered by Dr. Creany Wong, Associate Director of HKU-CTC, followed by a tour around the Centre.
The students reflected that it was an eye-opening experience as they had never thought of the possibility of entering the research field after the completion of a medical degree. Some considered that it could also be another way to contribute to the society and to the betterment of mankind.

-
HKU/QMH completed NMPA re-inspection and received clinical trials accreditation in a total of 14 clinical specialties/areas25th June, 2019
National Medical Products Administration (NMPA, previously called China Food and Drug Administration) visited Queen Mary Hospital on 29th April, 2019 for re-inspection on 14 clinical specialties (including HKU Phase 1 Clinical Trials Centre, cardiology, endocrinology & metabolism, respiratory medicine, hepatobiliary & pancreatic surgery and liver transplantation, obstetrics & gynaecology, haematology & bone marrow transplantation, neurology, nephrology, clinical immunology, orthopaedics & traumatology, gastroenterology & hepatology, oncology and paediatrics & adolescent medicine). The overall inspection result was satisfactory and the said specialties successfully received the continuing accreditation by the NMPA, meaning that the clinical drug trial data from these specialties are acceptable by the NMPA for evaluation of new drug approval applications.
HKU/QMH has been accredited by the NMPA for conducting clinical drug trials in six clinical specialties since 2006. HKU Phase 1 Clinical Trials Centre and seven additional clinical specialties were further accredited in July 2016.


-
HKUMed shines in latest QS University rankings: Top 30 globally and top 3 in Asia!29th April, 2019
The LKS Faculty of Medicine, The University of Hong Kong (HKUMed), through the outstanding quality of its research, teaching and innovation, emerged as top 30 globally and ranked top 3 in Asia!
The latest edition of the QS World University Rankings by Subject 2019, released recently by global higher education analyst Quacquarelli Symonds, saw HKUMed achieving its highest rank since 2016, with its place rose from 34th last year to 29th this year.
The subject rankings are compiled using four components with different weightings applied for each subject, namely academic reputation (40%), employer reputation (10%), research citations per paper (25%) and H-index (25%). HKUMed has obtained the high indicative score of 84.3 and performed very strongly on all four criteria, which demonstrated its all-rounded achievements.
HKUMed has consistently performed well in world rankings. Apart from the QS World Rankings, at the Times Higher Education World University Rankings 2019 released earlier last year, HKUMed also was ranked as 29th worldwide in the clinical, pre-clinical and health subject, which again recognized its outstanding performance.
HKU-CTC, together with HKUMed, will continue to work hand-in-hand to create a better future for mankind.

-
Chinese New Year Booth at Queen Mary Hospital30th January, 2019
HKU-CTC set up a Chinese New Year Booth at Queen Mary Hospital on 30th January, 2019 to share the joy and happiness with the public. Information panels with introduction of clinical trials were displayed. Other than that, Dr. Hong, one of the CTC Panda Ambassadors, visited the booth on that day. He gave out red packets and fai-chuns to the supporters. It attracted many visitors to come and learn more about clinical trials. Here are some highlights of the event:



-
Secondary school students visited the HKU Phase 1 Clinical Trials Centre21st November, 2018
On November 21, 2018, a group of 20 students from St. Paul’s Co-educational College visited HKU Phase 1 Clinical Trials Centre. Following a presentation of the concept of clinical trials by HKU-CTC’s representative, the students had the opportunity to have a tour around the Centre. The students were briefed on the facilities of the Centre and its unique features. They were all impressed by how they are designed to adhere to the highest international standard to safeguard patients’ rights and well-being.

-
The 8th Annual Meeting of World Federation of Chinese Medicine Societies (WFCMS) Committee of Ethics Review (世界中醫藥學會聯合會倫理審查委員會第八屆學術年會)18th November, 2018
The World Federation of Chinese Medicine Societies (WFCMS) is a non-profit organization established in 2003 in China with a mission to facilitate knowledge exchange in Traditional Chinese Medicine (TCM) among academic institutions worldwide. Its Committee of Ethics Review is dedicated in fostering standard of ethics review in TCM clinical research, and has launched the first government recognized accreditation scheme for research ethics review system in 2015, namely CAP. As of today, ethics committee from 43 National 3A TCM and multi-disciplinary hospitals in the Chinese Mainland attained CAP accreditation.
Attributable to the robust ethics review mechanism in Hong Kong, representative of HKU-CTC was invited to speak in its 8th Annual Meeting held in Guangzhou on 17-18 November 2018 to share the environment of ethics review in Hong Kong, and to introduce its innovative “Six-dimensional Ethics Review Evaluation Tool” to the audience. The annual meeting attracted more than 160 ethics professionals to attend.

-
The 3rd Seminar on Safety Evaluation in Drug Clinical Trials (第三屆藥物臨床試驗安全性評價研討會)18th November, 2018
Subject protection is one of the core principles under ICH GCP. To safeguard this important principle, proper collection of safety information from clinical trials and performing comprehensive safety evaluation are utmost important, in particular to novel immuno-oncology drugs as this is an innovative area in oncology but the safety profile of the drugs still needs continuous evaluation through clinical research.
On 17-18 November 2018, the China Anti-Cancer Association organized its ‘3rd Seminar on Safety Evaluation in Drug Clinical Trials’ in Guangzhou, which attracted more than 150 oncology experts from hospitals, academic institutions and industry to participate. Representative of HKU-CTC elaborated on the current situation and challenges in safety evaluation in drug clinical trials, and its systematic evaluations and innovative points of view have drawn the attention of all participants with positive feedbacks received.


-
2018 Annual Meeting of Chinese Pharmaceutical Association Research Committee for Drug Clinical Evaluation cum Clinical Research Forum (2018年中國藥學會藥物臨床評價研究專業委員會學術年會暨臨床研究高峰論壇)11th November, 2018
Quality with efficiency is the primary objective of the National Medical Products Administration (NMPA) (formerly named CFDA) in the past few years started with the 722 storm in China in 2015. Many new regulatory requirements and guidelines relating to clinical research were implemented thereafter, resulted in upsurge demand for efficient model for clinical research management and oversight in academic institutions or hospitals.
In response to this hot area, Chinese Pharmaceutical Association invited representatives of HKU-CTC to share its successful experience in developing an authentic Site Management Organization (SMO) in managing clinical research in HKU/QMH at its ‘2018 Annual Meeting of Chinese Pharmaceutical Association Research Committee for Drug Clinical Evaluation cum Clinical Research Forum’ on 10-11 November 2018 in Guangzhou. More than 400 participants from various backgrounds, including government officials, study site personnel and industry partners attended this event and the success of HKU-CTC’s SMO model has drawn much attention of different clinical trials stakeholders after the forum.


-
HKU-CTC 20th Anniversary Ceremony & Gala Dinner7th November, 2018
HKU-CTC’s 20th Anniversary Ceremony and Gala Dinner was successfully held on 7th November, 2018. The event was the grand finale of a series of anniversary activities during 2018. Some 200 prominent officials, academia, healthcare practitioners, clinical research professionals and industrial representatives joined us in reviewing HKU-CTC’s 20 years of ground-breaking journey in clinical research and gave their heartfelt greetings in Loke Yew Hall, a historic landmark of HKU.
Professor Karen Lam, Chairman of HKU-CTC, kick-started the ceremony by expressing her gratitude to all the stakeholders in clinical research during the welcoming address. Our guests of honour, Professor Sophia Chan, Secretary for Food and Health of the HKSAR Government, and Professor Paul Tam, Provost and Deputy Vice-Chancellor of HKU, highlighted the importance of clinical research to healthcare and scientific advancement and congratulated HKU-CTC’s contributions to the Hong Kong society and worldwide over the past 20 years.
A highlight of the evening was the interactive and interesting panel discussion featured by five distinguished HKU clinical researchers including (by alphabetical order of their last names) Professor Tak-Mao Chan, Professor Bernard Cheung, Professor Ching-Lung Lai, Professor Yu-Lung Lau and Professor Hextan Ngan, who together reviewed the history of clinical research over the past decades and shared their insights on the future research trends and opportunities for Hong Kong.
The society’s support is the key to the success of clinical research. We want to express our sincere gratitude to Baron Junior Music Academy, Theatre Cokida and Gingko House’s The Beginners Band – who showed their strong support to clinical research on behalf of different generations of Hong Kong people.
For more photos of the event, please visit https://20anniversary.ctc.hku.hk.


-
The 4th International Clinical Trial Center Network (ICN) Symposium cum 4th Annual Steering Board Meeting13th September, 2018
The 4th ICN symposium was successful held in Istanbul, Turkey on 13 September 2018 by one the founding members, Center of Excellence for Clinical Research of University of Istanbul. The symposium highlighted in investigator-initiated clinical research and data sharing. Representatives of ICN shared their local laws, regulations, as well as institutional policies relating to data sharing across countries.
Followed by the symposium, the 4th annual steering board meeting was successfully held at a beautiful off-site venue of Istanbul University on 14 September 2018. Representative of HKU-CTC was invited to do an opening remark by sharing its insights, experiences and current interactions with partners in Mainland China. Various topics, including overview of ICN memberships and operations team, legal and governance aspects, future ICN strategies and ICN outlooks were discussed. In addition, Dr. Thomas Hiemstra of Cambridge Clinical Trials Unit was elected as the new ICN Vice-Chairman during the meeting.


-
Practical Workshop on Clinical Trials Principles and Practice for Investigators and Clinical Research Personnel for Hong Kong Sanatorium & Hospital11th August, 2018
Hong Kong Sanatorium & Hospital (HKSH) invited HKU-CTC to organize a one-day practical workshop on clinical trials principles and practice investigators and clinical research personnel for its staff who are interested in conducting clinical research. More than 60 HKSH staff from various clinical departments attended the workshop and all of them enjoyed the interactive case studies designed by HKU-CTC and positive feedbacks were received.


-
Queen Mary Hospital: Best Hospital in the Greater Bay Area and Top-3 in the Chinese Community!18th July, 2018
In a recent survey “2017 Best 50 Hospitals in the Guangdong-Hong Kong-Macau Greater Bay Area” conducted by Asclepious Healthcare consultant, Queen Mary Hospital (“QMH”), a teaching hospital of The University of Hong Kong (“HKU”), ranked first amongst the rest! The ranking was carried out among comprehensive hospitals and traditional Chinese medicine hospitals in nine cities and two special administrative regions in the Greater Bay Area. The assessment criteria of the survey covered four aspects, including medical expertise, resources, hospital operation and academic impact. QMH’s application of advanced medical technologies and its active participation in research are important factors for QMH to gain its first place. In addition, QMH is also ranked a top-3 hospital in the Chinese Community in Asclepious survey “2017 Best 100 hospitals in the Chinese Community”.
HKU-CTC has a long history of collaboration with QMH. Since our establishment in 1998, we have been supporting the professional research teams of HKU and QMH in managing their clinical research projects in QMH. With this encouraging recognition as the best hospital in the area, HKU-CTC will continue to strive in advancing human healthcare by promoting, attracting and facilitating clinical studies at the highest professional standards.

-
Visit from Hong Kong Biotechnology Organization (HKBIO)29th June, 2018
A group of 23 HKBIO members paid a visit to HKU Phase 1 Centre on June 29, 2018. Managing Director of HKU-CTC introduced HKU-CTC through a lively presentation and a fruitful discussion was conducted among the members. It is envisage that this first contact will open unlimited collaborative opportunities between the HKBIO members and our Centre in the future.


-
Visit from ICN Founding Member – University Hospital Zurich28th June, 2018
Dr. Jürg Lustenberger, the Head of Quality Management of University Hospital Zurich Clinical Trial Center visited HKU-CTC on June 28, 2018. Dr. Lustenberger shared his experience on GxP inspections and quality management of clinical trials with colleagues of HKU-CTC. He also paid a visit to our dedicated Pharmacokinetic Laboratory and Phase 1 Centre, and was impressed by the well thought-out quality management system being implemented.



-
Visit from Shenzhen PKU-HKUST Medical Center15th June, 2018
Three guests from Shenzhen PKU-HKUST Medical Center visited HKU-CTC for knowledge exchange on June 15, 2018. Managing Director of HKU-CTC presented an overview of HKU-CTC, and an interactive discussion was conducted. They highly valued the layout of HKU-CTC’s website and the informative contents contained therein in particular, which facilitated the reading by clinical research stakeholders and even the general public.

-
10th DIA China Annual Meeting24th May, 2018
The 10th DIA China Annual Meeting was successfully held on May 23-25, 2018 in Beijing International Convention Center. This annual meeting focused on the recent and upcoming transformational changes in the innovation and regulatory environment of the Chinese Mainland, and has achieved a record high of more than 2,300 participants from the pharmaceutical industry, government and academic institutions attended.
As an active player in advocating clinical trials management in the Chinese Mainland over the past few years, HKU-CTC’s Managing Director was honoured to be invited by the Steering Committee to give two presentations in the annual meeting. On May 24, 2018, Mr.Henry Yau, our Managing Director gave a lecture on ‘Experience Sharing on Development of HKU Phase 1 Clinical Trials Centre’ in the Diamond Session - Hospital Presidents Forum, which participated by more than 400 attendees. The interactive discussion during the forum brought a lot of innovative ideas and was highly valued by the participants. In addition to this highlighted lecture, he also shared his view on ‘How to Ensure Adequate Adverse Event (AE) Reporting from Investigator/Clinical Trial Oversight Perspective’ in a Session entitled Clinical Trial AE Reporting—from Collection, to Processing, Analysis and Summary, to Authority Review on May 25, 2018. The 200 participants were attracted by the insightful opinions proposed by our Managing Director on analyzing the reasons of under reporting of AEs by investigators.



-
Visit from Market and Quality Supervision Commission of Shenzhen Municipality20th May, 2018
Ms. Xiana Wang, the Counsel of Market and Quality Supervision Commission of Shenzhen Municipality led a delegation of 11 people visited HKU-CTC on May 20, 2018. Ms. Wang assured the efforts HKU-CTC had rendered over the years and its dedication to facilitating clinical trial management in the Chinese Mainland. The delegation was also impressed by the development of our Phase 1 Centre, which expedited the registration of novel drugs by the CFDA through contributions of clinical trial data in the Chinese population.


-
Visit from National Cheng Kung University Hospital8th May, 2018
A delegation from the National Cheng Kung University Hospital Clinical Trial Center (NCKUH CTC) visited HKU-CTC on May 8, 2018. Managing Director of HKU-CTC introduced the clinical research management model of HKU-CTC and a fruitful and interactive discussion was conducted. They also paid a visit to Phase 1 Centre and were impressed by the sophisticated facilities and acknowledged the efficient operational workflows there implemented.

-
Guangdong-Hong Kong-Macau Phase 1 Clinical Research Joint Platform(粵港澳 I 期臨床研究聯合平台)5th May, 2018
Innovation and Technology is one of the key highlights under the Guangdong-Hong Kong-Macau Bay Area (“Greater Bay Area”) collaborative platform, whereas medical healthcare and biotechnology industry is anticipated to contribute to the success of this initiative.
Attributable to the tremendous demand on high quality phase 1 clinical research centre in the Mainland China in recent years, a robust open platform for knowledge exchange in phase 1 clinical research is of great importance. With this, HKU-CTC was invited to be one of the hosting organizations of the “Guangdong-Hong Kong-Macau Phase 1 Clinical Research Joint Platform” (“Joint Platform”) to share its international experience in managing phase 1 clinical research and assisting in the development of phase 1 clinical research centre in the Greater Bay Area.
The inauguration ceremony of the Joint Platform was officiated by the representatives of Guangdong Pharmaceutical Association and government officials of the Guangdong province in Guangzhou on 05 May 2018, followed by an introductory presentation on the mission of the Joint Platform by Professor Zhongyuan Xu. Representatives of HKU-CTC were honoured to give a duet presentation on “8 Questions 8 Answers - Phase 1 Clinical Research Centre Development and Operations”, which attracted positive feedbacks from over 150 participants from various cities in the Greater Bay Area.


-
Visit from Dongguan People’s Hospital20thApr, 2018
5 guests from the Dongguan People’s Hospital visited our Phase 1 Clinical Trials Centre on April 4, 2018. It was a great opportunity to share the development of clinical trials in Mainland China and it was a fruitful discussion.

-
2018年新書推薦 ─ 醫與研4th Apr, 2018
醫與研,從來密不可分。
《醫與研》收錄33篇由香港大學與瑪麗醫院醫學教授、專科醫生與科研人員撰寫的文章,深入淺出地介紹本港及世界各地的醫學科研人員如何透過臨床研究,跟疾病對決,為保障人頪的健康而努力。
策劃: 香港大學臨床試驗中心 出版: 健康動力有限公司 出版日期: 2018年3月 ISBN: 978-988-12429-6-9 《醫與研》已於三聯書店及商務印書館有售! 如欲索取更多資訊,歡迎致電2255 2550或電郵至ctcentre@hku.hk.


-
The 4th International Conference on Phase 1 and Early Phase Clinical Trials (ICPOEP 2018) was successfully held on 1-2 March, 2018.6th Mar, 2018
The 4th International Conference on Phase 1 and Early Phase Clinical Trials (ICPOEP 2018) was successfully held on 1-2 March, 2018 at Hyatt Regency Hong Kong, Tsim Sha Tsui in Hong Kong. Around 350 participants have joined the event.
-
HKU-CTC is going to hold the 4th International Conference on Phase 1 and Early Phase Clinical Trials (ICPOEP 2018) during 1-2 March, 2018 in Hong Kong. The theme for this year is Drug Development in the New Era: A Global Perspective. Please visit the conference website www.icpoep.com for more details.16th Jan, 2018
-
HKU-CTC’s new article is available in the Health Action Magazine (January 2018 Issue)9th Jan, 2018
-
‘What is Generic Drugs?’ video produced by Clinical Trials Centre, The University of Hong Kong (HKU-CTC) was aired!9th Jan, 2018
Everyone of us should have taken generic drugs before, but how much do you know about this. HKU-CTC produced a short video named ‘What is Generic Drugs’ to introduce the concept of generic drugs and bioequivalent study to the general public, and was aired on Facebook on December 30, 2017. Dr. Tommy Cheung (Deputy Medical Director, HKU Phase 1 Centre) together with our lovely kids jointly talk about a story on generic drugs in this video.
The video can be viewed on Facebook or YouTube.

-
HKU-CTC’s new article is available in the Health Action Magazine (December 2017 Issue)4th Dec, 2017
-
HKU-CTC’s new article is available in the Health Action Magazine (November 2017 Issue)10th Nov, 2017
-
The Facebook of Clinical Trials Centre, The University of Hong Kong (HKU-CTC) Goes LIVE!!!7th Nov, 2017HKU-CTC’s Facebook goes live TODAY on November 7, 2017. Please like and share our Facebook link to your family members, friends and colleagues who are interested in healthcare information and medical research. Stay tuned to our posts and we are here to answer your inquiries on Facebook. Facebook Link: https://www.facebook.com/hkuctc/
-
China Forums of Clinical Research Capacity Building and Human Research Participants Protection (中国临床研究能力提升与受试者保护高峰论坛)7th Nov, 2017
-
Clinical Research Forum on Coping Strategies to the New Trend in China (新形势下我国临床试验应对策略高峰论坛)1st Nov, 2017
-
The 3rd International Clinical Trial Center Network (ICN) Symposium cum 3rd Annual Steering Board Meeting1st Nov, 2017
-
Seminar on New Drug Development after CFDA Joining ICH (CFDA加入ICH后的新药临床试验研讨会)1st Nov, 2017
-
China Heart Congress (CHC) 2017 and the 2nd China Vascular Congress (CVC) 20171st Nov, 2017
-
HKU-CTC’s new article is available in the Health Action Magazine (October 2017 Issue)6th Oct, 2017
-
HKU-CTC’s new article is available in the Health Action Magazine (September 2017 Issue)11th Sep, 2017
-
HKU-CTC’s new article is available in the Health Action Magazine (August 2017 Issue)7th Aug, 2017
-
The History, Vision and Future of HKU-CTC: An Interview with Pharma Boardroom24th Jul, 2017
During an interview with the journalist of Pharma Boardroom, a premier electronic report in healthcare and science industry, Mr. Henry Yau, HKU-CTC’s Managing Director shared his view on centre’s development since its establishment in 1998 - From how it grows from a small unit staffed by 3 to 4 people to a professional team with over 50 staff, evolves from an administration supporting unit to an one-stop SMO and CRO centre facilitating all kinds of clinical trials, as well as extends its support from local study sites to international multi-centre clinical studies. Scope of HKU-CTC's services, its history of development and vision and the value of clinical trials in Hong Kong were also discussed.
Full article could be accessed at Pharma Boardroom or downloaded here.
-
Seminar on Ethics Issues and the Governance of Innovative Clinical Research (创新临床研究的伦理问题与管理研讨会)21st Jul, 2017
-
Seminar on Phase 1 Centre Development and Management (一期药物临床试验室建设与管理培训班)6th Jul, 2017
-
HKU-CTC’s new article is available in the Health Action Magazine (July 2017 Issue)5th Jul, 2017
-
HKU-CTC’s new article is available in the Health Action Magazine (June 2017 Issue)8th Jun, 2017
-
HKU-CTC’s new article is available in the Health Action Magazine (May 2017 Issue)29th May, 2017
-
HKU-CTC’s new article is available in the Health Action Magazine (April 2017 Issue)24th Apr, 2017
-
HKU-CTC’s new article is available in the Health Action Magazine (March 2017 Issue)14th Mar, 2017
-
HKU-CTC’s new article is available in the Health Action Magazine (February 2017 Issue)1st Feb, 2017
-
HKU-CTC’s new article is available in the Health Action Magazine (January 2017 Issue)6th Jan, 2017
-
HKU-CTC’s new article is available in the Health Action Magazine (December 2016 Issue)12th Dec, 2016
-
HKU-CTC’s new article is available in the Health Action Magazine (November 2016 Issue)17th Nov, 2016
-
The 3rd International Conference on Phase 1 and Early Phase Clinical Trials (ICPOEP 2016) was successfully held on 3-4 November, 2016.15th Nov, 2016
The 3rd International Conference on Phase 1 and Early Phase Clinical Trials (ICPOEP 2016) was successfully held on 3-4 November, 2016 at Hyatt Regency Hong Kong, Tsim Sha Tsui in Hong Kong. More than 300 participants have joined the event. The event photos could be viewed at http://www.icpoep.com/photoalbum/ConfFoto.
-
HKU-CTC’s new article is available in the Health Action Magazine (October 2016 Issue)20th Oct, 2016
-
HKU-CTC is going to hold the 3rd International Conference on Phase 1 and Early Phase Clinical Trials (ICPOEP 2016) during 3-4 November, 2016 in Hong Kong. Please visit the conference website www.icpoep.com for more details.15th Sep, 2016
-
HKU-CTC’s Volunteer Resource Centre (“VRC”) is conducting a survey to collect public opinions on clinical trial participation.15th Aug, 2016
-
HKU Phase 1 Clinical Trials Centre received official clinical trials accreditation by the China Food and Drug Administration (CFDA) on July 20, 20162nd Aug, 2016

The HKU Phase 1 Clinical Trials Centre (HKU Phase 1 Centre), together with seven clinical specialties of its affiliated teaching hospital – Queen Mary Hospital (QMH), received official accreditation by the China Food and Drug Administration (CFDA) for conducting clinical drug trials. The HKU Phase 1 Centre is a dedicated, state-of-the-art clinical research facility of HKU Clinical Trials Centre (HKU-CTC), specifically designed for performing phase 1, early phase and clinical pharmacology trials. This accreditation signifies that research data and results arising from clinical trials conducted in the HKU Phase 1 Centre and the seven clinical specialties will be accepted by the CFDA for evaluation of applications for drug registration in the Mainland China, strengthening Hong Kong’s special position as a hub for drug research and development in China and worldwide.
Clinical Drug Trial Accreditation by the CFDA

Clinical trials are essential for medical advancement and are subject to stringent ethical and regulatory governance. Since 2006, HKU/QMH has been accredited by the CFDA for conducting clinical drug trials in seven clinical specialties (including anaesthesiology, cardiology, endocrinology & metabolism, haematology & bone marrow transplantation, hepatobiliary & pancreatic surgery/liver transplantation, obstetrics & gynaecology, and respiratory medicine). With the new accreditation for the HKU Phase 1 Centre and seven additional clinical specialties (including clinical immunology, gastroenterology & hepatology, nephrology, neurology, oncology, orthopaedics & traumatology, and paediatrics & adolescent medicine), HKU/QMH now is accredited by the CFDA in a total of 15 areas.
The accreditation for the HKU Phase 1 Centre is of exceptional significance as there was no official accreditation for any phase 1 centre in China.
Success of the HKU Phase 1 Centre
Drug development is a lengthy process involving drug discovery, pre-clinical research and phase 1 to 3 clinical trials. Phase 1 clinical trials constitute the earliest stage of investigation of novel drugs in research participants – whether patients or healthy volunteers – and represent a critical step towards practical advancement in medicine.

“The HKU Phase 1 Centre was established in 2014 with the support of the Food and Health Bureau and the Hospital Authority of Hong Kong. In a short period of only two years, the Centre has already completed eight trials. Now there are seven trials ongoing, and another 15 trials being planned. The major research areas include cancers, hepatitis, haematology and kidney diseases. The official accreditation by the CFDA represents the recognition of our clinical research works in the HKU Phase 1 Centre, adding strong momentum to Hong Kong’s clinical trial activities and drug research and development. Without doubt, we will see an upsurge in the volume of phase 1, early phase and clinical pharmacology trials here,” says Professor Karen Lam Siu-ling, Rosie T T Young Professor in Endocrinology and Metabolism, Chair Professor of Department of Medicine and Chairman of HKU-CTC, Li Ka Shing Faculty of Medicine, HKU.
“Patient safety and drug safety are our top priorities. We now have the excellent facilities and a dedicated team to evaluate new drugs safely. This is tremendous news for Hong Kong and our country,” says Professor Bernard Cheung Man-yung, Sun Chieh Yeh Heart Foundation Professor in Cardiovascular Therapeutics, Clinical Professor of Department of Medicine and Medical Director of HKU Phase 1 Centre, Li Ka Shing Faculty of Medicine, HKU.
Advancement in Medicine through Clinical Trials

“HKU/QMH has long been a leading institution in clinical trials, with outstanding reputation both locally and internationally. Over the years, HKU-CTC has developed into a professional clinical trial management organisation capable of running and supporting local and multinational clinical trials of various kinds. Accreditation by the CFDA is a significant acknowledgement of our clinical trials capabilities, in terms of clinical sciences, research ethics and research quality. With the joint effort of our clinical investigators, research institutions, the pharmaceutical industry and the HKSAR Government, and of course also the great support by our research participants, we are confident that medicine and healthcare will continue to advance and the public will be benefited,” says Professor Yu-lung Lau, Doris Zimmern Professor in Community Child Health, Chair Professor of Department of Paediatrics and Adolescent Medicine and Chief Director of HKU-CTC, Li Ka Shing Faculty of Medicine, HKU.
About HKU-CTC
HKU-CTC is an academic clinical research organisation established under Li Ka Shing Faculty of Medicine, HKU in 1998, and is the central platform for management and coordination of clinical trials under HKU/QMH. It provides one-stop solutions for clinical research programmes – whether locally in Hong Kong or of a multinational scale. In collaboration with HKU’s expert investigators, local and overseas research institutions and the medical research industry, its professional team supports planning and management of various clinical trials – from designing research protocols, identifying suitable research participants, coordinating research procedures, collecting and analysing research data, to final reporting and publication of research results. Over the past 18 years, HKU-CTC has already facilitated over 1,100 clinical trials. HKU Phase 1 Centre was established by HKU-CTC in 2014 for performing phase 1, early phase and clinical pharmacology trials.
HKU-CTC has a robust governance and management system. Policies and strategies are directed by its Committee of Management (COM) with wide representation by the senior management of HKU/QMH. The COM now consists of 12 members including a Chairman, Dean and Associate Dean (Research) of the Medical Faculty, Hospital Chief Executive of QMH, Directors of HKU-CTC and other key stakeholders in clinical trials. The entire HKU-CTC now has a staff of 50 full-time clinical trial professionals covering a full spectrum of clinical trial expertise.
Enroll as Clinical Trial Participants
Any person who wishes to participate in clinical trials and support drug research and development, please visit HKU-CTC’s website (http://www.hkuctc.com/phase1_enroll_zh.php) for more details and registration.
-
HKU-CTC’s new article is available in the Health Action Magazine (July 2016 Issue)20th Jul, 2016
-
HKU-CTC’s new article is available in the Health Action Magazine (June 2016 Issue)13th Jun, 2016
-
HKU-CTC’s new article is available in the Health Action Magazine (May 2016 Issue)17th May, 2016
-
HKU-CTC’s new article is available in the Health Action Magazine (Apr 2016 Issue)12th Apr, 2016
-
Collaboration between The University of Hong Kong Clinical Trials Centre and the Affiliated Hospital of Guizhou Medical University7th Apr, 2016

HONG KONG and GUIYANG, GUIZHOU – 7th Apr, 2016 – The University of Hong Kong Clinical Trials Centre (HKU-CTC) and the Affiliated Hospital of Guizhou Medical University (GMUAH) today announced the establishment of a strategic collaboration on management of clinical trials in China. The objective is to develop a clinical study site management organization (SMO) model for facilitating the management and operation of international-standard clinical trials whilst fitting the specific local environment of China. This collaboration brings together the global clinical trial management experience of HKU-CTC and GMUAH’s local expertise in clinical trial operations, and is anticipated to contribute to the further advancement of international clinical trials and new drug development in the country.
“Protection of the rights and safety of research participants and safeguarding the quality of research data are the two core considerations in clinical trials. Over the past decades, the relevant standards have been getting more and more stringent – whether globally or locally. Enforcing compliance with such requirements has become an increasing challenge for clinical research centres and research personnel worldwide in terms of organizational management, human resources and financial resources. There is a pressing need for a practical solution, and a robust SMO model that supports full compliance in an efficient and sustainable manner is deemed the right way to go. Since its establishment in 1998, HKU-CTC has been investing major efforts in designing and developing a SMO model that supports the management and operation of international-standard clinical trials, and it has been proven a successful model through real implementation under HKU during the past 18 years. GMUAH is a well-established clinical research organization that has solid knowledge and experience in conducting clinical trials in China. Collaboration between GMUAH and HKU-CTC will integrate the expertise of the two parties, giving birth to a SMO model matching the practical needs of the country,” says Henry Yau, Managing Director and Honorary Assistant Professor of HKU-CTC.

“Development of the healthcare and innovation industries is a strategic direction of the Guizhou Province. Supporting novel drug research and development, including building clinical trial capabilities, aligns with this direction. Guizhou Medical College is dedicated to medical education and research, and GMUAH has been recognized as a national clinical drug trial institution by the China Food and Drug Administration (CFDA) since 2009. Over the years, we have been focusing on developing a professional platform for supporting clinical trials. We are very glad to collaborate with HKU-CTC, an internationally reputable clinical research management organization. I am confident that our common vision and complementary expertise will make a big success in developing a suitable SMO model that adapts to local needs and also contributes to the advancement of the healthcare and innovation industries in Guizhou and the whole country,” says Professor He Yan, Director of the GCP Office of GMUAH.
About HKU-CTC: HKU-CTC is a full-service academic clinical research management organization established under The University of Hong Kong since 1998, and is dedicated to promoting and facilitating international-standard clinical research in the region. Its novel SMO model is known to be effective for professional clinical study site management and has been taken as reference by hospitals/universities in Asian countries.
About GMUAH: GMUAH was founded in 1941 and is one of the earliest higher medical education institutions in the southwest region of China. It is now the largest 3A integrated hospital in Guizhou, providing a full range of medical services including clinical care, medical education, medical research, emergency services, rehabilitation and preventive medicine. Since 2009, GMUAH has been accredited by the CFDA as a national clinical drug trial institution in 15 clinical specialties.
Contacts:
Henry Yau
Managing Director &
Honorary Assistant Professor
Clinical Trials Centre
The University of Hong Kong
kcyau@hku.hk
www.HKU-CTC.comProfessor Yan HE
Director & Professor
GCP Office
Affiliated Hospital of Guizhou Medical University
physicianheyan@126.com
gcp.gmcah.com -
HKU-CTC’s new article is available in the Health Action Magazine (Mar 2016 Issue)29th Mar, 2016
-
HKU-CTC’s new article is available in the Health Action Magazine (Feb 2016 Issue)2nd Mar, 2016
-
HKU-CTC’s new article is available in the Health Action Magazine (Jan 2016 Issue)12th Jan, 2016
-
HKU-CTC’s new article is available in the Health Action Magazine (Dec 2015 Issue)8th Dec, 2015
-
The 2nd International Conference on Phase 1 and Early Phase Clinical Trials (ICPOEP 2015) was successfully held on 20 - 21 November, 2015.27th Nov, 2015
The 2nd International Conference on Phase 1 and Early Phase Clinical Trials (ICPOEP 2015) was successfully held on 20 - 21 November, 2015 at Hyatt Regency Hong Kong, Tsim Sha Tsui in Hong Kong. About 340 delegates from five continents attended the conference and showed ideas about phase 1 and early phase clinical trials and drug development on an internatonal horizon.
Aligned with the theme of “New Drug Research for Tomorrow – In Asia and Worldwide”, our 18 distinguished speakers have brought a wide range of perspectives from their remarkable presentations. The panel discussion and Q&A sessions also attracted active discussions among speakers, session chairs, panelists and delegates by enthusiastic audience.
Please click here to visit the conference website.
-
HKU-CTC’ Biennial Report 2013/2014 is available.27th Nov, 2015
-
HKU-CTC’s new article is available in the Health Action Magazine (Nov 2015 Issue)18th Nov, 2015
-
HKU-CTC’s new article is available in the Health Action Magazine (Oct 2015 Issue)12nd Oct, 2015
-
HKU-CTC’s new article is available in the Health Action Magazine (Sep 2015 Issue)21st Sep, 2015
-
HKU-CTC launches the new interface of HKU Clinical Trials Registry (HKUCTR)27th Aug, 2015
-
HKU-CTC’s new article is available in the Health Action Magazine (Aug 2015 Issue)6th Aug, 2015
-
The Pearl Report on clinical trials in Hong Kong was aired28th Jul, 2015
On July 27, 2015, the Pearl Report, produced by TVB Pearl, about clinical trials in Hong Kong was aired.
The programme reviewed the recent development of clinical trials in Hong Kong, with special focus on phase 1 clinical trials. Renowned clinical investigators including Professor Karen Lam (Chairman, HKU-CTC), Professor Yu-lung Lau (Chief Director, HKU-CTC), Professor Bernard Cheung (Medical Director, HKU Phase 1 Centre), Dr. Tommy Cheung (Deputy Medical Director, HKU Phase 1 Centre) and Dr. Joanne Chiu (Deputy Medical Director, HKU Phase 1 Centre) explained the value and challenges of conducting clinical trials. Professor Sydney Tang (Chairman, HKU/HA HKW IRB) and Dr. Derrick Au (Director, Quality & Safety, Hospital Authority) illustrated the stringent research ethics review and approval mechanism. The importance of developing Hong Kong's clinical trial capabilities was highlighted by Professor Sophia Chan (Under Secretary for Food and Health, HKSAR) and Ms. Sabrina Chan (Executive Director, HKAPI). Volunteers in clinical trials also explained why they participated, and openly shared their experience in those trials.
The program will be broadcasted again at 11:35 am on 1st August, 2015 (Saturday), and is also available now online at http://programme.tvb.com/news/pearlreport/episode/20150727/.
-
HKU-CTC’s new article is available in the Health Action Magazine (Jul 2015 Issue)15th Jul, 2015
-
HKU-CTC’s new article is available in the Health Action Magazine (Jun 2015 Issue)16th Jun, 2015
-
HKU Phase 1 Clinical Trials Centre moves forward to its 1st Anniversary Focus on phase 1 and early phase clinical trials to bring novel treatments3rd Jun, 2015
In order to support clinical trials on novel drugs for various kinds of diseases, The University of Hong Kong Phase 1 Clinical Trials Centre (Phase 1 Centre) has been established last year. The Phase 1 Centre is a dedicated, state-of-the-art clinical research facility of HKU Clinical Trials Centre (HKU-CTC), specifically designed for conducting phase 1 and early phase clinical trials. With this advanced establishment, more high impact clinical trials will be conducted in the future and more novel treatments will emerge to combat different diseases – for the well-being of Hong Kong citizens and the mankind.
Importance of HKU Phase 1 Centre
“The Phase 1 Centre is a world-class clinical research facility with advanced equipment and a leading clinical, scientific and management team. It allows our investigators to conduct phase 1 and early phase clinical trials that were not possible in the past, and is going to be a powerhouse boosting novel drug research with results finally benefiting our patients and the public,” says Professor Karen Lam Siu-ling, Chairman of HKU-CTC and Rosie T T Young Professor in Endocrinology and Metabolism, Chair Professor and Head of Department of Medicine, Li Ka Shing Faculty of Medicine, HKU.
“The Phase 1 Centre is an ideal facility for clinical investigators to conduct clinical trials, in particular drug trials that require close monitoring. The team in the Phase 1 Centre is highly professional, not only in conducting research activities but more importantly in caring of our research participants,” says Professor Yuen Man-fung, Li Shu Fan Medical Foundation Professor in Medicine, Chair Professor of Gastroenterology and Hepatology, Department of Medicine, Li Ka Shing Faculty of Medicine, HKU.
Unique Value of Phase 1 Centre
Phase 1 and early phase clinical trials constitute the early stage of investigation of novel drugs in research participants – whether patients or healthy volunteers – and represent a critical step towards practical advancement in medicine. Such trials usually involve intensive and timely clinical assessments, diagnoses, blood sampling and testing, and commonly require continuous monitoring of trial participants – over 24 hours or even longer. Stringent quality control processes must also be implemented to ensure proper performance of each research procedure in compliance with research protocols, and also with local and international regulatory requirements. Without a dedicated, fully equipped and staffed facility, such trials could not be optimally carried out.
Achievements of Phase 1 Centre
Since the commencement of operation of the Phase 1 Centre in mid-2014, it has already supported three novel drug trials, in cancer and hepatitis. Currently four trials are ongoing there, and 12 other trials are under planning. The main research areas include cancers, hepatitis, immunology, diabetes and infectious diseases. So far over 70 participants have enrolled in the completed and ongoing trials. With the rapid advancement in medical research locally and worldwide, the number of phase 1 and early phase clinical trials conducted in the Phase 1 Centre is expected to keep growing in the years to come, and more new treatments will emerge.
Background of HKU-CTC
HKU-CTC is an academic clinical research organisation established under Li Ka Shing Faculty of Medicine, HKU in 1998, and is the central platform for management and coordination of clinical trials under HKU and its affiliated teaching hospital – Queen Mary Hospital (QMH).
HKU-CTC provides one-stop solutions for clinical research programmes – whether locally in Hong Kong or of a multinational scale. In collaboration with HKU's expert investigators, local and overseas research institutions and the medical research industry, its professional team supports planning and management of various clinical trials – from designing research protocols, identifying suitable research participants, coordinating research procedures, collecting and analyzing research data, to final reporting and publication of research results. Over the past 17 years, HKU-CTC has already facilitated over 1,000 clinical trials, covering oncology, cardiovascular diseases, gastroenterology & hepatology, endocrinology, diabetes & metabolism, haematology and other clinical specialties.
HKU-CTC has a robust governance and management system. Policies and strategies are directed by its Committee of Management (COM) with wide representation by the senior management of the Medical Faculty and QMH. The COM now consists of 12 members including a Chairman, Dean and Associate Dean (Research) of the Medical Faculty, Hospital Chief Executive of QMH, Directors of HKU-CTC and other key stakeholders in clinical trials.
The entire HKU-CTC now has a staff of 40 full-time clinical research management professionals under its six functional teams, including its Phase 1 Centre Operations Team, Pharmacokinetics Laboratory Operations Team, Business & Project Acceleration Team, Project Management & Contract Services Team, Quality Assurance Team and Corporate Services & Administration Team.
Enroll as phase 1 clinical trial participants
If you wish to participate in phase 1 clinical trials and support research and development of novel drugs, please visit HKU-CTC's website (http://www.hkuctc.com/phase1_enroll_zh.php) for more details and registration.
For press photos and powerpoint presentation, please visit the below link:
http://www.med.hku.hk/v1/archives/12518 -
HKU-CTC’s new article is available in the Health Action Magazine (May 2015 Issue)22nd May, 2015
-
HKU-CTC is going to hold the 2nd International Conference on Phase 1 and Early Phase Clinical Trials (ICPOEP 2015) during 20-21 November, 2015 in Hong Kong. Please visit the conference website www.icpoep.com for more details.8th May, 2015
-
HKU-CTC’s updated New Project Form - for Sponsored Clinical Studies is available for download15th Apr, 2015
-
HKU-CTC’s new article is available in the Health Action Magazine (Apr 2015 Issue)15th Apr, 2015
-
HKU-CTC’s new article is available in the Health Action Magazine (Feb 2015 Issue)9th February, 2015
-
Successful Registration of PRACTISE® Trademark with Trade Marks Registry Intellectual Property Department, HKSAR5th January, 2015
Clinical Trials Centre, The University of Hong Kong (“HKU-CTC”) – 5th January 2015 – HKU-CTC today announced that the trademark of PRACTISE® has been successfully registered with the Trade Marks Registry Intellectual Property Department, HKSAR on 5th January 2015 since its application made on 17th January 2014.
With the mission of advancing human healthcare by promoting, attracting and facilitating clinical studies, HKU-CTC is devoted to enhancing the quality of clinical studies by providing training to clinical investigators and study sites personnel under its proprietary training program - PRACTISE® (Professional Research Accreditation for Clinical Trials Investigative Site Executives). Over the last few years, PRACTISE® program or its individual modules has gained wide recognition by medical institutions and regulatory authorities in the Asian, Middle East and North African regions, including Hong Kong, Macau, Taiwan, mainland China, Malaysia, Vietnam, UAE and Egypt, which attracted the attendance of more than 1,000 participants.
“Our PRACTISE® program provides an interactive and intensive environment for efficient and effective learning and has been welcomed by many clinical research personnel from different regions. With the certification of registration of the PRACTISE® trademark, the intellectual property right of HKU-CTC will be better protected as it will keep the momentum to deliver PRACTISE® workshops in other countries in the coming years.” says Dr. Creany Wong, Co-chief Trainer of PRACTISE®.
About PRACTISE®: PRACTISE® is a comprehensive training program specifically designed for addressing the needs of clinical investigators, study coordinators and other study site personnel. The program includes 25 modules covering three key areas including (a) clinical trial concepts and compliance; (b) clinical trial preparation; and (c) clinical trial process. For details, please refer to http://www.hkuctc.com/knowledge_practise.php.
Contact:
Dr. Creany Wong
Co-chief Trainer, PRACTISE®
Clinical Trials Centre
The University of Hong Kong
creanywo@hku.hk
 Professional Research Accreditation for Clinical Trials Investigative Site Executives
Professional Research Accreditation for Clinical Trials Investigative Site Executives -
HKU-CTC’s new article is available in the Health Action Magazine (Jan 2015 Issue)5th January, 2015
-
HKU-CTC’s new article is available in the Health Action Magazine (Dec 2014 Issue)15th December, 2014
-
HKU-CTC’s new article is available in the Health Action Magazine (Nov 2014 Issue)10th November, 2014
-
HKU-CTC’s new article is available in the Health Action Magazine (Oct 2014 Issue)12th October, 2014
-
HKU-CTC launches its Learning Management System (LMS)8th October, 2014
8th October, 2014 – The Clinical Trials Centre of The University of Hong Kong (HKU-CTC) announced the launch of its tailored Learning Management System (LMS) today.
Being a leading academic clinical research organization, HKU-CTC adopts the concept of total quality management (TQM). HKU-CTC’s LMS: (i) provides investigators, clinical research personnel and HKU-CTC’s staff a convenient access to a secure central platform for current policies, standard operating procedures (SOPs) and other quality documents via the internet anytime, anywhere; (ii) enhances compliance through close tracking of each individual’s training progress; and (iii) supports convenient documentation of completed training through maintenance of training records and generation of individual training transcripts.
It is anticipated that the LMS will enhance quality and compliance management in HKU-CTC and for clinical research at HKU and its teaching hospital (Queen Mary Hospital).
Functionalities of HKU-CTC’s LMS
- Secure central online platform for accessing the current policies, SOPs and relevant forms and document templates of HKU-CTC and study sites
- Training assignment and management, including training on internal policies/SOPs and external training courses/events
- Submission and endorsement of training reports
- Online examination on Good Clinical Practice (GCP) and clinical research ethics
- Maintenance of training records and generation of individual training transcripts
-
HKU-CTC’s new article is available in the Health Action Magazine (Sep 2014 Issue)12th September, 2014
-
Collaboration between TRREE and HKU-CTC in e-learning1st Apr, 2014
HONG KONG and Neuchâtel, Switzerland – 1st April, 2014 – The Clinical Trials Centre of The University of Hong Kong (HKU-CTC) and the Training and Resources in Research Ethics Evaluation Programme (TRREE) of the University of Neuchâtel, Switzerland announced formation of an international strategic collaboration today. The objective is to promote the principles of ethics and regulations of health and medical research involving human participants to research personnel, especially those in Asia, Middle East and North Africa. This collaboration brings together the research ethics expertise of the West and the East and integrates TRREE’s on-line e-Learning with HKU-CTC’s on-site training, and is anticipated to contribute significantly to protection of research participants and quality of human research in the Asian & MENA region and also globally.
Under the collaboration, HKU-CTC will contribute directly to the maintenance and future development of TRREE, and integrate TRREE into its on-site clinical research training programme. HKU-CTC will also work together with TRREE for attaining a better recognition of TRREE as a high-quality training programme on Good Clinical Practice (GCP) within the clinical research community and the pharmaceutical industry, while maintaining its independence and strong academic position. In addition, HKU-CTC will develop a Hong Kong Local Supplement for TRREE, which will provide direct access to Hong Kong’s human research legislation, regulations and ethical standards.
“Protection of research participants and quality management are two major elements of human research ethics. Over the years, HKU-CTC has been putting much effort in promoting research ethics and GCP. Our on-site training programme, namely PRACTISE®, provides an interactive and intensive environment for efficient and effective learning. TRREE, a high-quality, open platform freely accessible to research personnel anytime anywhere through the Internet, offers a desirable mode of continuous learning. We are proud to collaborate with TRREE, and trust the integration of TRREE’s on-line platform and HKU-CTC’s on-site training programme will create enormous synergy in promoting research ethics and GCP in the Asian & MENA region as well as globally,” says Henry Yau, Managing Director of HKU-CTC.
“Safeguarding research ethics is a continuous mission that requires international collaboration. I am very glad to know that HKU-CTC shares the same vision and has been making its effort in the Asian & MENA region which houses the biggest world population and plays an increasingly important role in human research. With our aligned vision and complementary expertise, I am sure this collaboration will bring solid value to the further development of research ethics in the region and also worldwide,” says Professor Dominique Sprumont, Coordinator of TRREE.
About TRREE: University of Neuchâtel (UniNE) is a public university in Neuchâtel, Switzerland. TRREE (www.trree.org) is an e-Learning and e-Resources platform initiated and coordinated by Professor Dominique Sprumont of the Institute of Health Law of UniNE for promoting the principles of human research ethics and regulations worldwide.
About HKU-CTC: HKU-CTC is a full-service academic research organisation established under The University of Hong Kong since 1998, and is dedicated to promoting and facilitating clinical research. Its proprietary on-site training programme – PRACTISE® (Professional Research Accreditation for Clinical Trials Investigative Site Executives) – is specifically designed for clinical investigators and research site personnel in the Asian & MENA region.
Contacts:
Henry Yau
Managing Director
Clinical Trials Centre
The University of Hong Kong
kcyau@hku.hk
www.HKU-CTC.comProfessor Dominique Sprumont
Coordinator, TRREE
Institute of Health Law
University of Neuchâtel
dominique.sprumont@unine.ch
www.trree.org -
HKU Phase 1 Clinical Trials Centre comes into operation1st February, 2014













